Data Listings Clinical Trials . guideline on computerised systems and electronic data in clinical trials. create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. This support may include activities related to. what is a clinical study report (csr)? the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic. This guideline replaces the 'reflection paper on. describes the organization that provides funding or support for a clinical study. this paper proposes a data listing programmed in sas and output in ms excel where in all patient data from different study domains is.
from nwflcrg.com
“integrated full report of an individual study of any therapeutic, prophylactic or diagnostic. guideline on computerised systems and electronic data in clinical trials. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. what is a clinical study report (csr)? This support may include activities related to. the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. this paper proposes a data listing programmed in sas and output in ms excel where in all patient data from different study domains is. This guideline replaces the 'reflection paper on. describes the organization that provides funding or support for a clinical study.
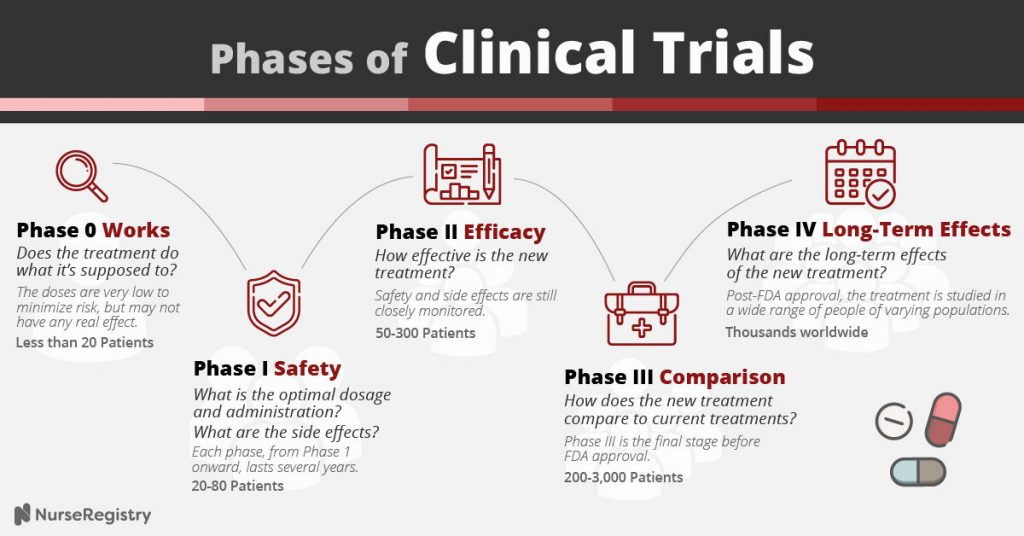
What are the different phases of a clinical trial?
Data Listings Clinical Trials describes the organization that provides funding or support for a clinical study. what is a clinical study report (csr)? describes the organization that provides funding or support for a clinical study. This support may include activities related to. create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. This guideline replaces the 'reflection paper on. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic. this paper proposes a data listing programmed in sas and output in ms excel where in all patient data from different study domains is. guideline on computerised systems and electronic data in clinical trials.
From www.pharmamirror.com
Unveiling the Future Key Trends in Clinical Trial Technology Pharma Data Listings Clinical Trials guideline on computerised systems and electronic data in clinical trials. what is a clinical study report (csr)? create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. This guideline replaces the 'reflection paper on. biopharmaceutical companies generally use tables, listing and. Data Listings Clinical Trials.
From brasddp.com
BRAS DDP Clinical Trials Bras DDP Data Listings Clinical Trials This guideline replaces the 'reflection paper on. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. This support may include activities related to. the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. guideline on computerised systems and electronic data in clinical trials. this. Data Listings Clinical Trials.
From www.origent.com
Clinical Trial Analysis Origent Data Sciences Data Listings Clinical Trials This support may include activities related to. This guideline replaces the 'reflection paper on. this paper proposes a data listing programmed in sas and output in ms excel where in all patient data from different study domains is. create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for,. Data Listings Clinical Trials.
From clinnovo.blogspot.com
Clinnovo News Data Flow During Clinical Trial Process Data Listings Clinical Trials guideline on computerised systems and electronic data in clinical trials. what is a clinical study report (csr)? the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. this paper proposes a data listing programmed. Data Listings Clinical Trials.
From www.hdruk.org
Clinical trials Day 2022 How data can make trials faster, more Data Listings Clinical Trials describes the organization that provides funding or support for a clinical study. what is a clinical study report (csr)? create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. the nihr innovation observatory (nihrio), based at newcastle university, has launched a. Data Listings Clinical Trials.
From www.altexsoft.com
Clinical Data Management Roles, Steps, and Software Tools AltexSoft Data Listings Clinical Trials This support may include activities related to. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic. This guideline replaces the 'reflection paper on. this paper proposes a data listing programmed in sas and output in ms excel where in all patient data from different study domains is. create tables, listings and figures (tfls) using. Data Listings Clinical Trials.
From clinnovo.blogspot.com
Clinnovo News Clinical Trial Data Analysis Using SAS Workflow Data Listings Clinical Trials describes the organization that provides funding or support for a clinical study. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. This support may include activities related to. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic. create tables, listings and figures (tfls) using. Data Listings Clinical Trials.
From cancergrace.org
Clinical Trials Helping the Search CancerGRACE Data Listings Clinical Trials create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. this paper proposes a data listing programmed in sas and output in ms excel where in all patient data from different study domains is. describes the organization that provides funding or support. Data Listings Clinical Trials.
From pharmaguidances.com
Phases Of Clinical Trials Data Listings Clinical Trials the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. describes the organization that provides funding or support for a clinical study. create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. guideline on computerised systems and electronic. Data Listings Clinical Trials.
From www.medidata.com
The Future of Clinical Trial Data Management Data Listings Clinical Trials “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic. guideline on computerised systems and electronic data in clinical trials. the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. describes the organization that provides funding or support for a clinical study. biopharmaceutical companies generally use tables, listing and figures. Data Listings Clinical Trials.
From www.smartsheet.com
All about Clinical Trial Data Management Smartsheet Data Listings Clinical Trials describes the organization that provides funding or support for a clinical study. This support may include activities related to. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic. This guideline replaces the 'reflection paper on. . Data Listings Clinical Trials.
From www.alzheimersresearchuk.org
Clinical trials Alzheimer's Research UK Data Listings Clinical Trials This guideline replaces the 'reflection paper on. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic. this paper proposes a data listing. Data Listings Clinical Trials.
From www.lancaster.ac.uk
Historical Clinical Trials Lídia André Data Listings Clinical Trials create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. This support may include activities related to. This guideline replaces the 'reflection paper on. this paper proposes a data listing programmed in sas and output in ms excel where in all patient data. Data Listings Clinical Trials.
From clinicalcrunch.com
Tables, listings and Figures (TLFs) Clinical Data Visualizations Data Listings Clinical Trials This support may include activities related to. what is a clinical study report (csr)? guideline on computerised systems and electronic data in clinical trials. describes the organization that provides funding or support for a clinical study. the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. create tables, listings and figures (tfls). Data Listings Clinical Trials.
From cancer.uillinois.edu
Newly activated UI Cancer Center clinical trials University of Data Listings Clinical Trials this paper proposes a data listing programmed in sas and output in ms excel where in all patient data from different study domains is. what is a clinical study report (csr)? create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. “integrated. Data Listings Clinical Trials.
From mrctcenter.org
clinical trial Clinical Research Glossary Data Listings Clinical Trials create tables, listings and figures (tfls) using r programming in your clinical trial data analysis, not as a replacement for, but rather as an alternative. This support may include activities related to. the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. “integrated full report of an individual study of any therapeutic, prophylactic or diagnostic.. Data Listings Clinical Trials.
From 3iap.com
Pharmaceutical Clinical Trials Data Visualization 3iap Data Listings Clinical Trials This guideline replaces the 'reflection paper on. guideline on computerised systems and electronic data in clinical trials. what is a clinical study report (csr)? the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. This. Data Listings Clinical Trials.
From www.researchgate.net
Sample size and basic demographic data in clinical trials included in Data Listings Clinical Trials describes the organization that provides funding or support for a clinical study. the nihr innovation observatory (nihrio), based at newcastle university, has launched a comprehensive. what is a clinical study report (csr)? biopharmaceutical companies generally use tables, listing and figures (tfls) to present data collected in clinical trials and their. This guideline replaces the 'reflection paper. Data Listings Clinical Trials.